The Science Behind LED Grow Lights – How they Work
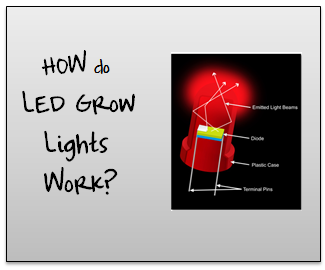
Welcome to this series on how LED grow lights work. The idea behind this series is to start from the ground up. If you can understand all the basics of an LED light then you’ll be able to make an informed decision about which grow light you should purchase. Note: In some parts of this series things get pretty technical. I do my best to explain everything as simply as possible. If you get confused and/or have a question just drop me a line in the comment box at the bottom of a post. Let’s get started!
How do LEDs produce light? The Basics of LEDs
In order to understand LED grow lights you are going to need to understand what diodes are (remember that LED stands for light emitting diode). In order to understand diodes you are going to need to understand semiconductors. The best place to start understanding semiconductors is with the most famous semiconductor, silicon.
Silicon is a Semiconductor
Silicon Powder Photo courtesy of Silane and Wikimedia
Silicon is always a great place to start when talking about semiconductors. Chances are you have heard about Silicon Valley and you know that silicon is used to make computer chips. Silicon isn’t really used in LED lights but it’s still a great place to start your understanding of these lights.
We all think we know what a semiconductor is, but I’m going to take a bit of time to review it here. A conductor is something that conducts electricity easily. An insulator is something that doesn’t conduct electricity well, and a semiconductor is somewhere in between (it’s a mediocre conductor of electricity).
Metals are great conductors because they have free electrons that can move between atoms. Silicon, by itself, actually doesn’t have any free electrons. It has 4 electrons in its outer orbit that are all bonded with other electrons in different atoms (this is called a covalent bond). This means that silicon doesn’t have any free electrons to pass the electricity around.
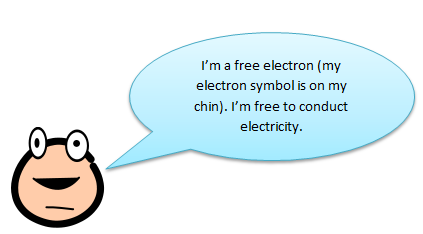
Free Electrons

Covalent Bond Electrons
Doping the Silicon
Yes I know that this is an LED grow light blog and I know what you think when I say the word doping, but I’m not talking about that here. Doping actually refers to the process of changing silicon from an insulator to a type of conductor known as a semiconductor.
There are two ways to dope silicon: N-Type doping and P-Type doping.
![]() Both types of doping are pretty straightforward. Remember when I said that silicon atoms each have four outer electrons that are all in covalent bonds? Well with N-Type (negative type) doping you add atoms (phosphorous or arsenic are usually the atoms of choice) that each have five outer electrons. This means that four of the electrons from the silicon and four of the electrons from the phosphorous or arsenic pair off, but one remains free!
Both types of doping are pretty straightforward. Remember when I said that silicon atoms each have four outer electrons that are all in covalent bonds? Well with N-Type (negative type) doping you add atoms (phosphorous or arsenic are usually the atoms of choice) that each have five outer electrons. This means that four of the electrons from the silicon and four of the electrons from the phosphorous or arsenic pair off, but one remains free!
P-Type (positive type) doping is very similar. Instead of using atoms with five outer electrons, you mix silicon with atoms that have three outer electrons (boron or gallium are the atoms of choice). This means that six of the electrons form a bond and one is free to pass electricity.
Positive and Negative (P-Type and N-Type)
I find all the positive and negative talk very confusing. In both P-Type and N-Type combinations you have one extra electron that is not bonded. What makes one positive and the other negative? I’m not sure anybody knows; sometimes scientists just have to choose a label and so they do.
The way I remember it is that with the N-Type doping you have nine total electrons and with P-Type doping you only have 7 total electrons. Electrons are usually considered negative so a -7 is less negative than a -9. Less negative equals positive or P-Type. Confused? Join the club.
In reality don’t worry too much if you are a bit confused at this point. It’s not that important that you understand all the labeling. The main takeaway from all of this talk is that by doping silicon you can turn it from an insulator (doesn’t pass electricity) to a semiconductor (that does pass electricity).
What is a Diode?
Okay, so we’ve talked about semiconductors, doping, and free electrons. What is a diode then? A diode is a combination of P-Type and N-Type semiconductors (more on this in a minute).
First, why would anyone want to combine P-Type and N-Type semiconductors? Well, a semiconductor by itself isn’t that cool. I mean sure it conducts electricity, but it doesn’t conduct electricity as well as a full-fledged conductor like metal will. The truth is a semiconductor by itself isn’t that cool.
However, when you combine P-Type and N-Type semiconductors you get something very, very cool. Something that all plants love: light. Remember that the purpose of this article is to explain how LED grow lights work.
How Does Combining P-Type and N-Type Semiconductors Create Light?
Now let’s discuss on combining semiconductors creates light. When you combine P and N-Type semiconductors and add a battery to the equation you have a diode!! (See the image below) Aren’t you glad you finally know what a diode is?
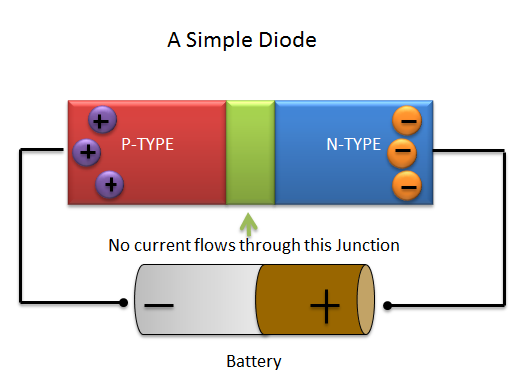
Batteries have a positive and a negative charge to them (you’ve probably seen these signs on batteries you’ve purchased. If the positive side of the battery is connected to the N-Type semiconductor (as in the picture above) then the electrons from the N-Type will move towards the battery (opposites attract). Similarly the P-Type electrons will move towards the negative charge of the battery. This forms a wide junction in the middle of the diode (pictured above in green).
However, when the battery is reversed (as in the picture below) the negative charge of the battery will push the N-Type electrons away and towards the P-type electrons. Similarly the positive charge of the battery will push the P-Type electrons towards the junction.
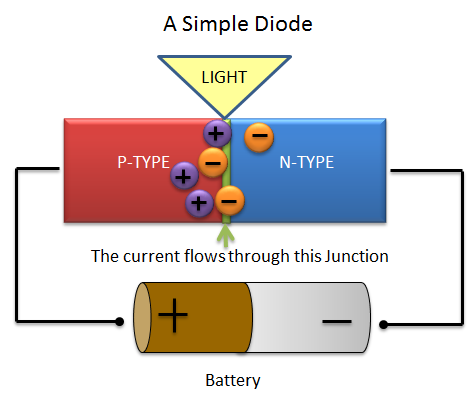
Amazingly enough when the N-type electrons collide with the P-type electrons light is created.
Remember that light is a bunch of small energy packets called photons. Photons are released from atoms when electrons move from a higher-energy orbit to a lower-energy orbit. When the N-Type electrons clash with the P-Type electrons they move from a higher-energy orbit to a lower-energy orbit and this releases the photons (light).
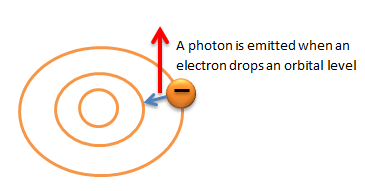
This is pretty cool stuff. It seriously amazes me that someone figured out how to put this all together.
Most LED Grow Lights aren’t Made of Silicon
Silicon diodes are very popular but the truth is they don’t put out much bright light. In the example above silicon is used to explain diodes, but in real life silicon isn’t used that much in the LED growing industry.
Any diode made up of semiconductors will have N-Type electrons that drop to a lower orbit. However, the type of semiconductors used will affect the amount of light produced. Some semiconductors have larger energy drops which mean more energy released and more light.
Remember that when N-Type electrons drop to a lower orbit photons will be released. However, small drops in orbit will result in low frequency photons (think infrared light here). Large drops in orbit will result in high-frequency photons (think ultraviolet). See the image below for further clarification.
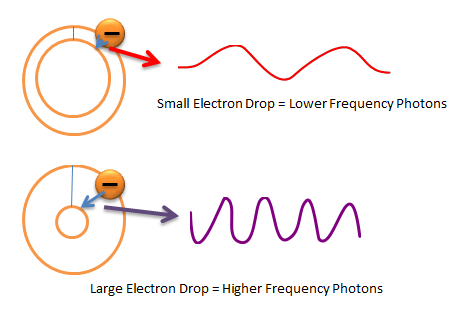
With silicon semiconductors when the N-Type electrons drop in orbit, the drop is very small. This means that silicon diodes produce low frequency infrared light. Some LED grow lights do have infrared light in them, but many do not.
For example, Cree (a popular LED manufacturer) uses semiconductor material made up of a mix of gallium nitride and indium nitride. This type of semiconductor produces a very bright light when compared to a silicon semiconductor.
Most LED grow lights emit light in the red and blue frequencies. This means that the diodes must be made up of semiconductor material that emits that type of frequency. It’s pretty amazing that scientists have figured out how to combine components in a way that will emit the proper light color or frequency.
FYI: Diodes that emit visible light are known as visible light emitting diodes or VLEDs.
Summary:
You might be a bit confused if this is your first time reading about semiconductors, N-Type and P-Type electrons, and diodes. Let’s do a quick summary of the key points.
- Semiconductors are insulators (like silicon) that have been changed so that they can conduct electricity. This process of changing an insulator to a semiconductor is known as doping.
- Scientists can create two types of semiconductors with doping: P-Type (positive) and N-Type (negative).
- When you combine P-Type and N-Type semiconductors with a battery you have a diode that can create light.
- Light is created because the N-type electrons fall from their outer orbit. When electrons fall, they lose energy. This energy is released in the form of photons (light).
- The farther the electron falls, the higher frequency of light the atom releases.
In the next section we’ll look at the inside of the LED and see where the diode is placed.


You must be logged in to post a comment.